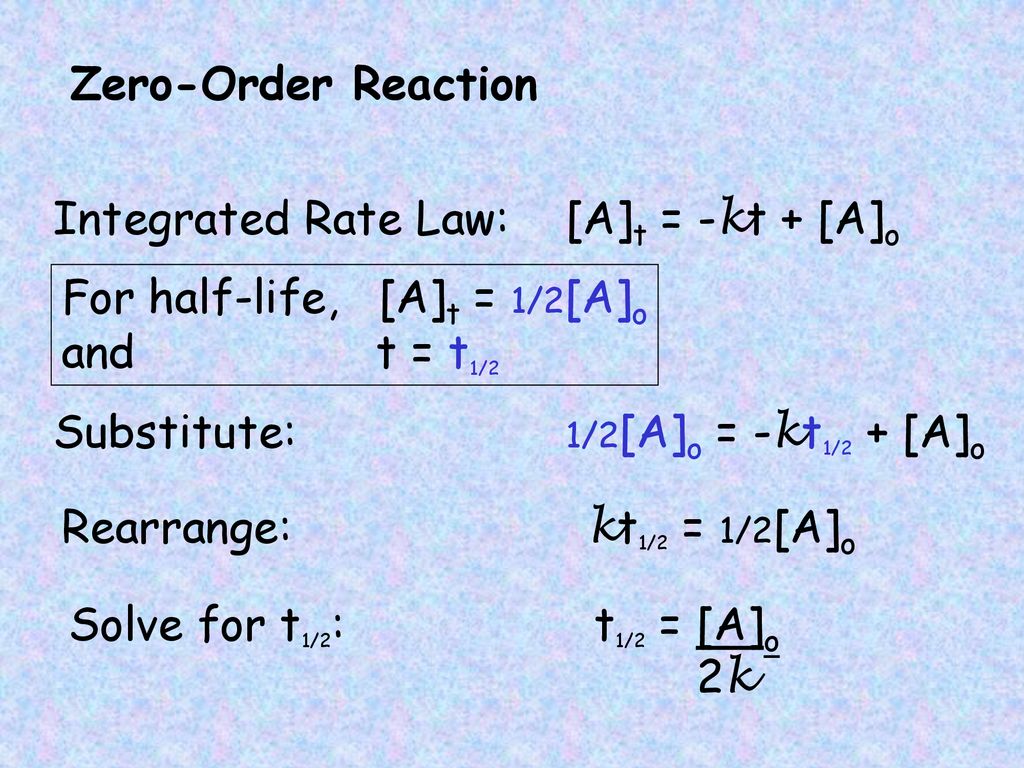
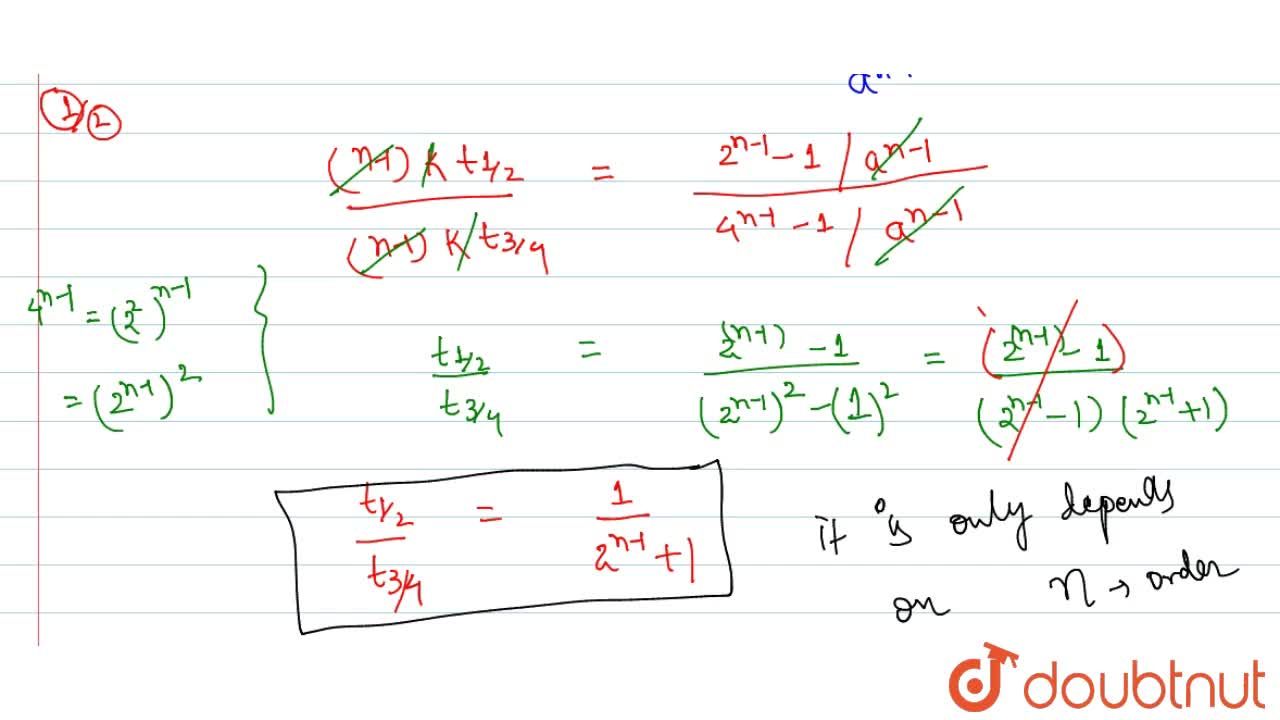
√ t1/2 for zero order reaction formula 226285-T1/2 for zero order reaction formula

Half Life Of A Second Order Reaction Derivation Youtube
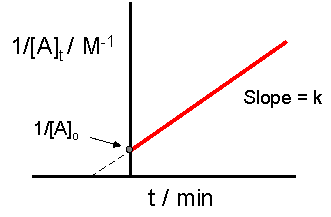
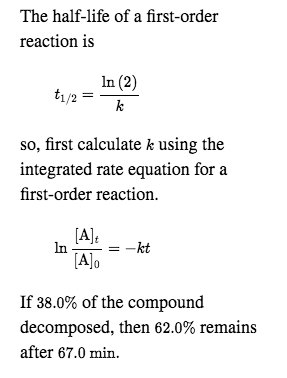
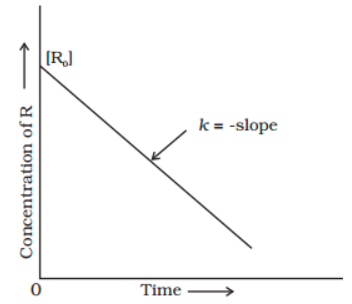
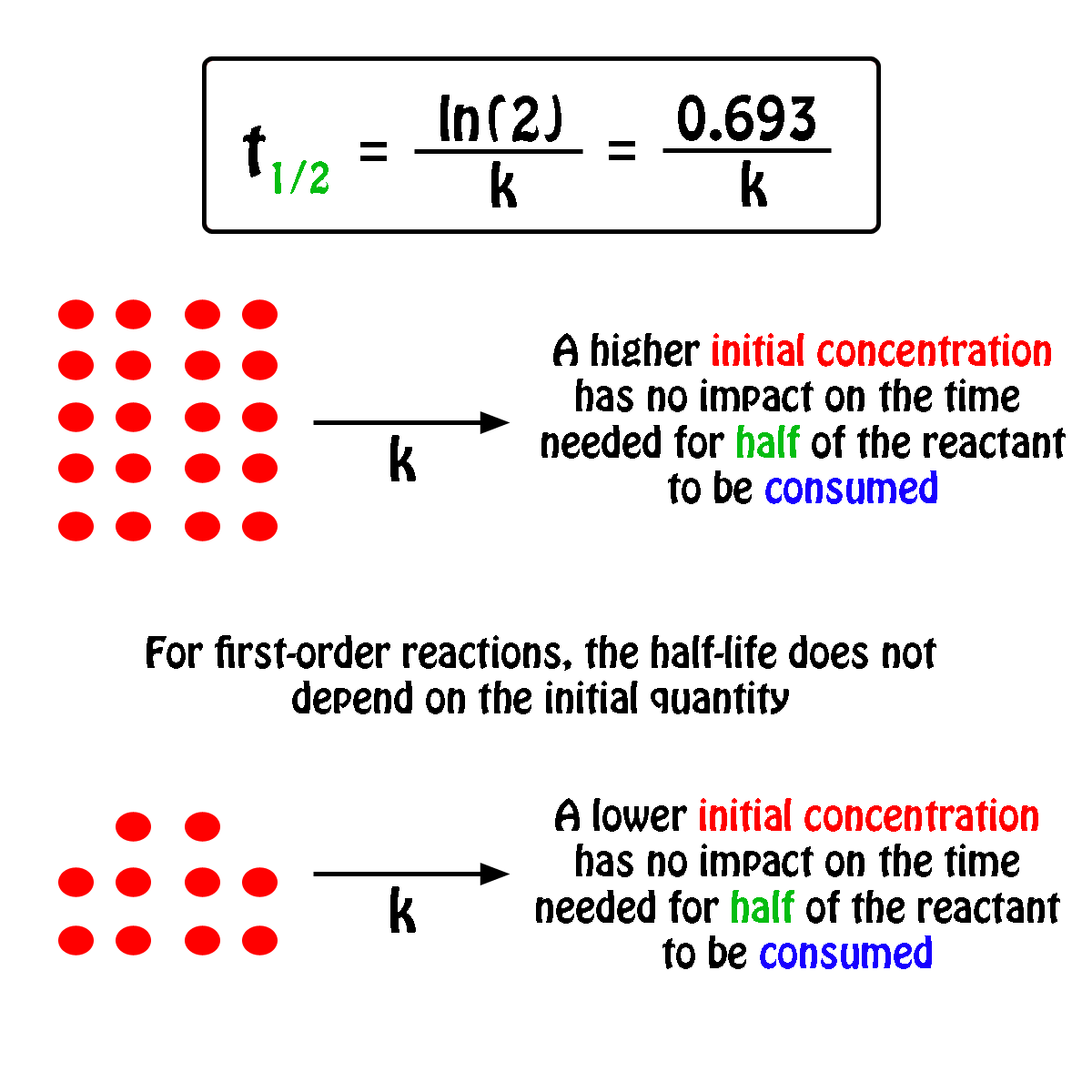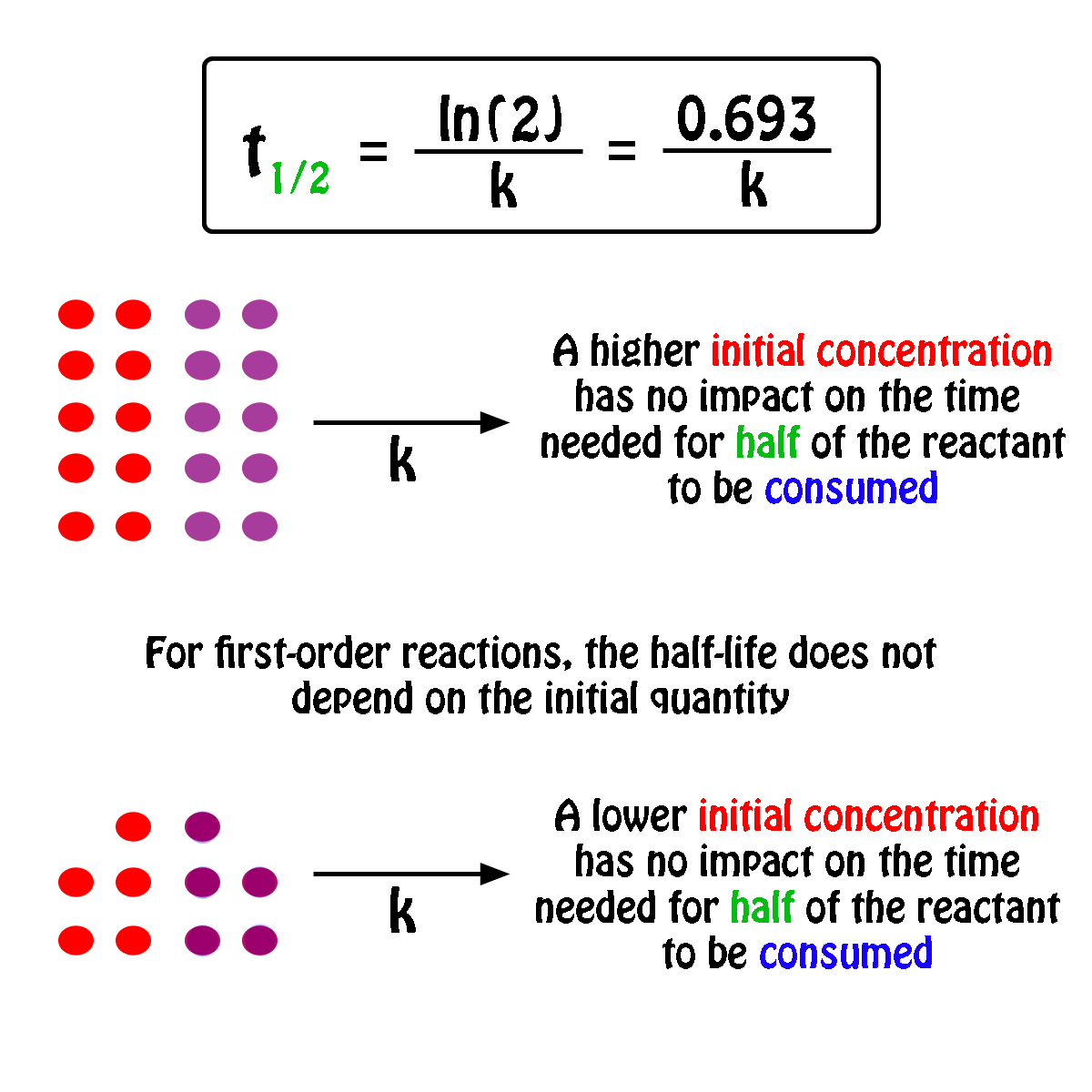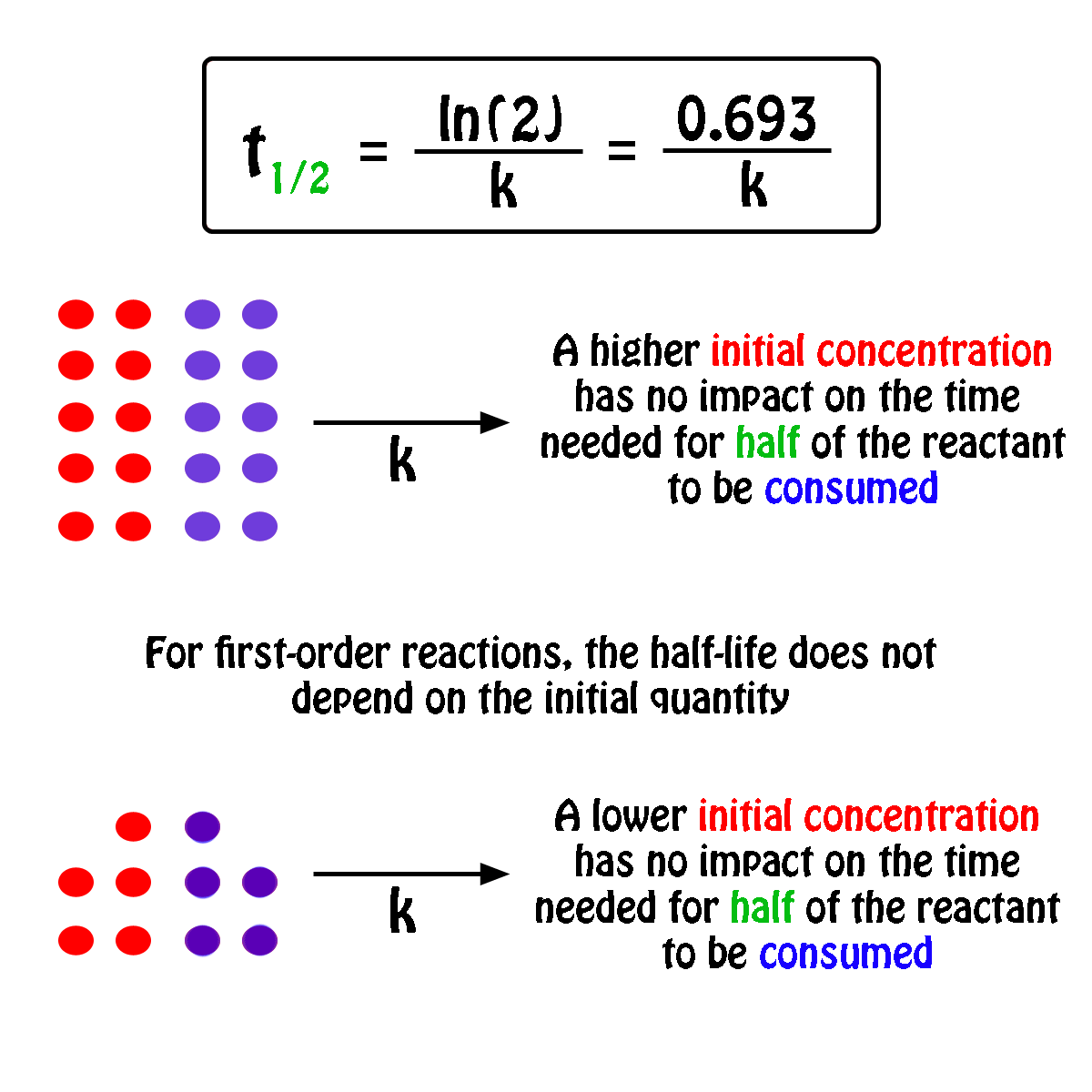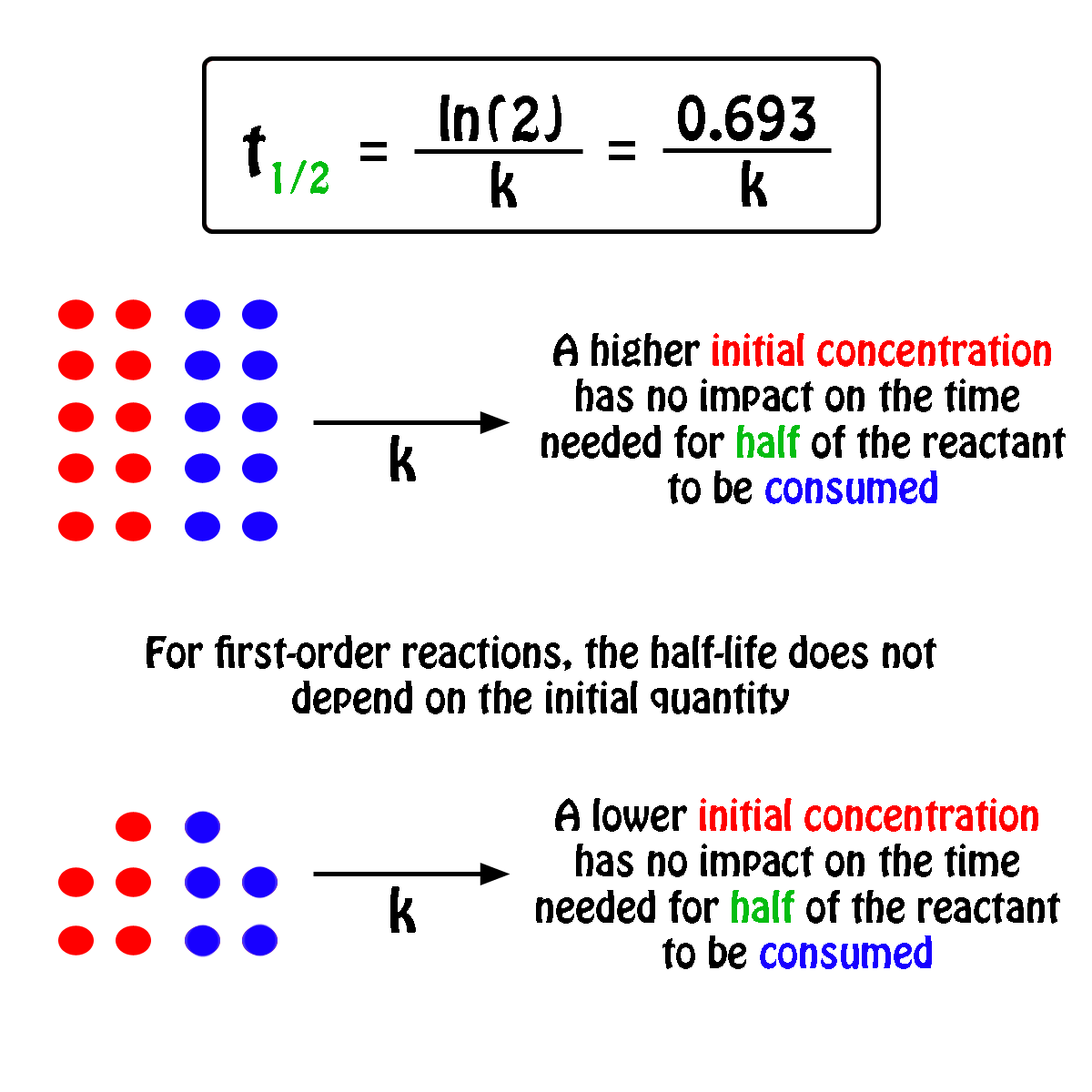
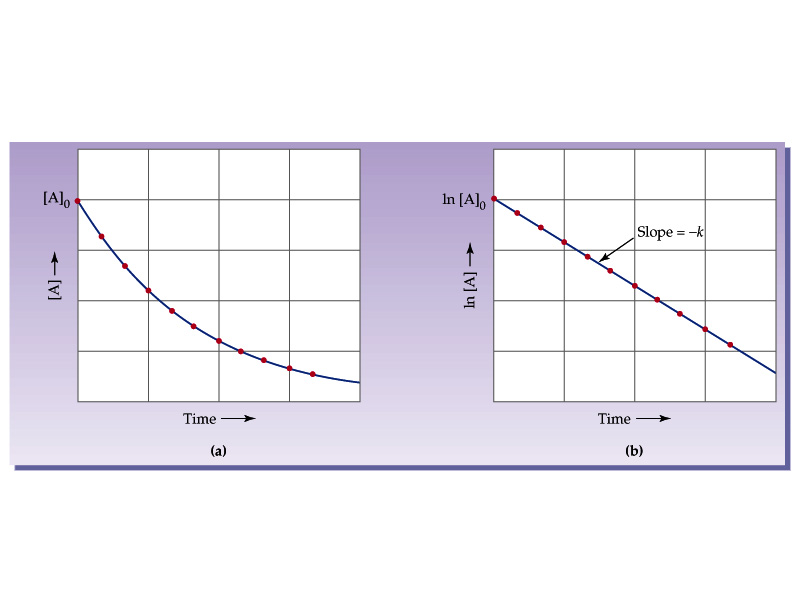
By rearranging this equation and using the properties of logarithms, we can find that, for a first order reaction t_\frac {1} {2}=\frac {ln (2)} {k} t21 = kln(2) What is interesting about this equationAnswer (1 of 2) 0 seconds(REFER PICTURE ATTACHED) Remember the formula between half life and other certain standard time of compleition of first order reaction=>REFER PICTURE
T1/2 for zero order reaction formula
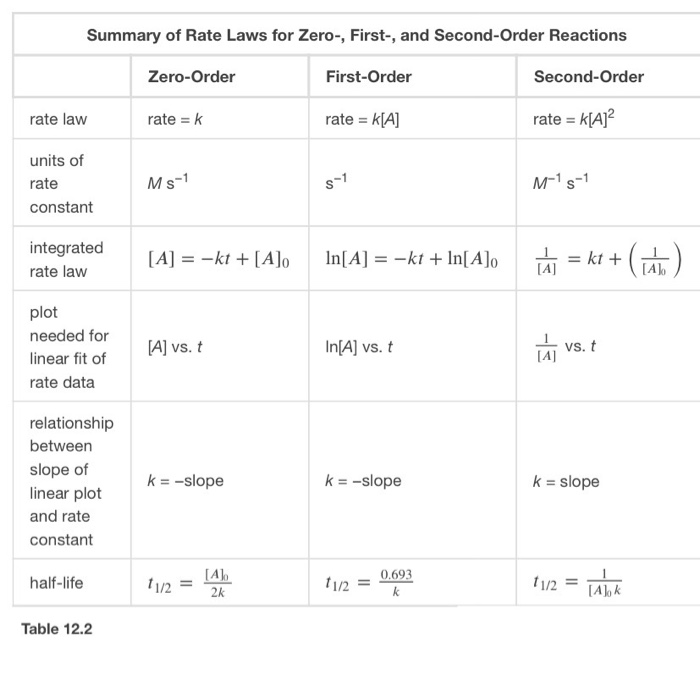
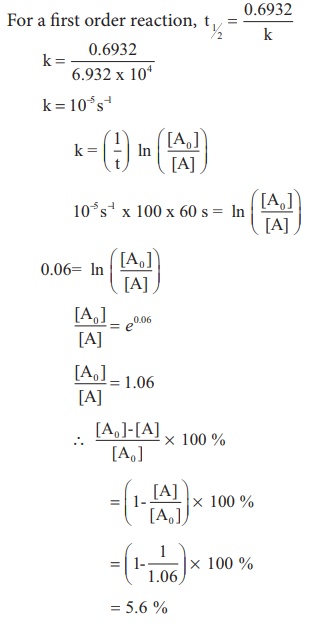
T1/2 for zero order reaction formula-For a firstorder reaction, the halflife is given by t 1/2 = 0693/k;Halflife equation for firstorder reactions t1/2=0693k t1/2=0693k where t1/2t1/2 is the halflife in seconds (s) (s), and kk is the rate constant in inverse seconds (s?1) (s?1) 1 To calculate the half

14 5 First Order Reactions Chemistry Libretexts
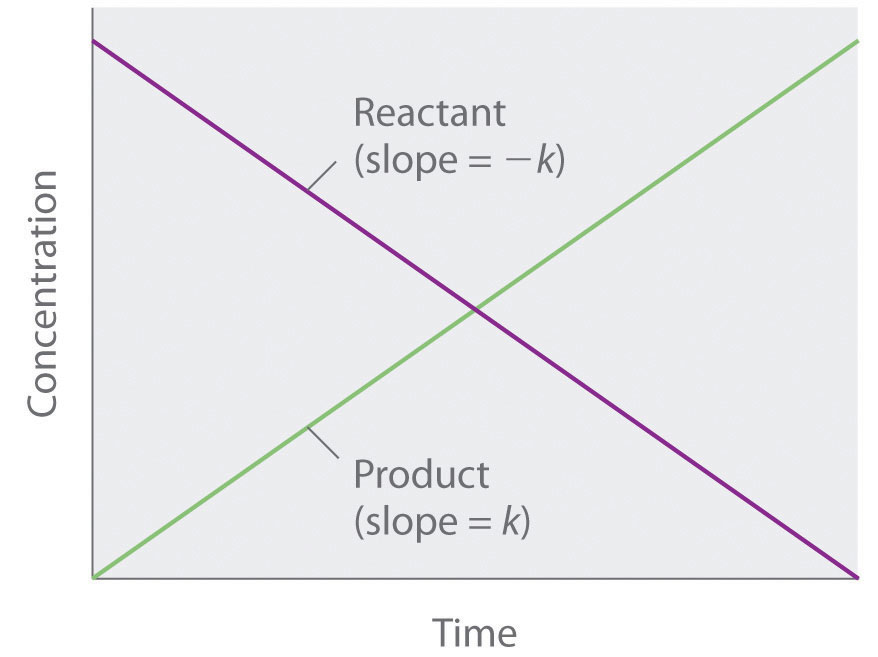
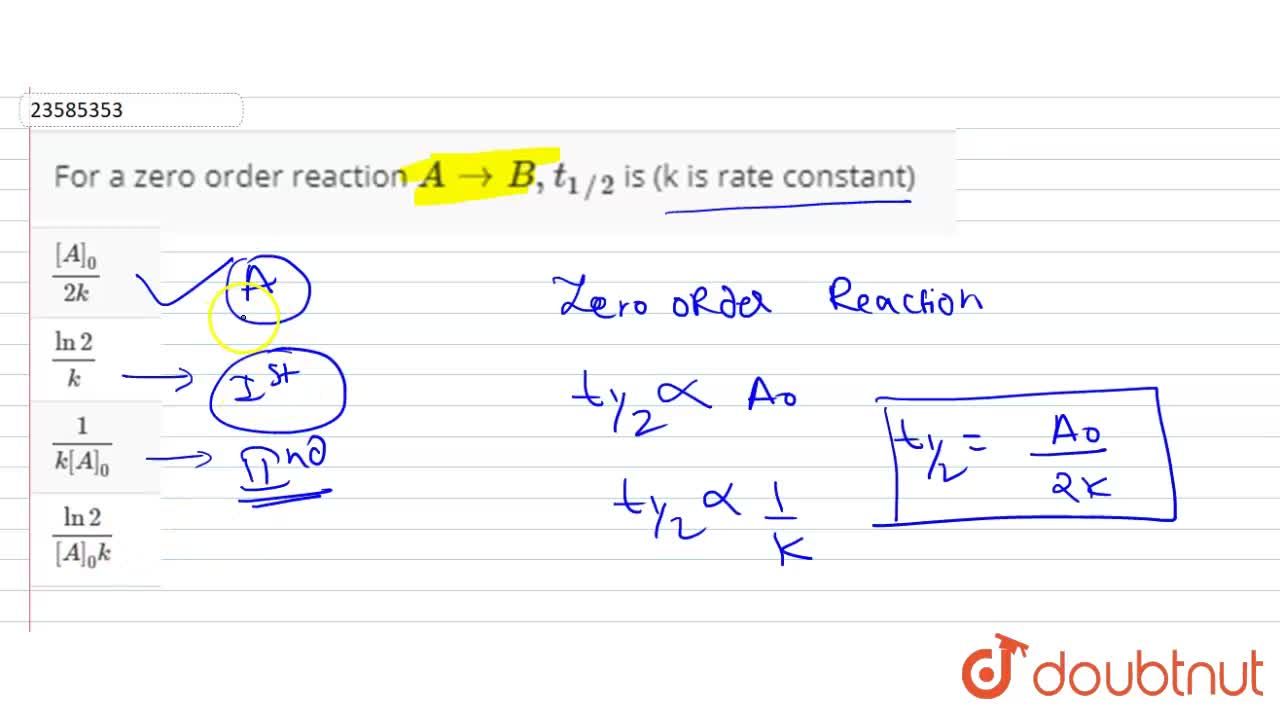
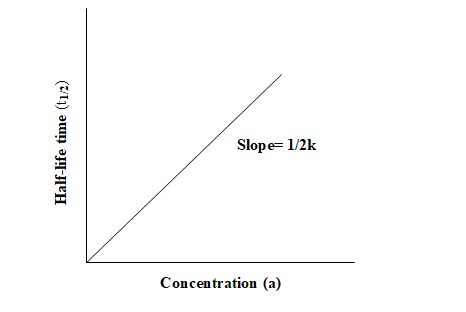
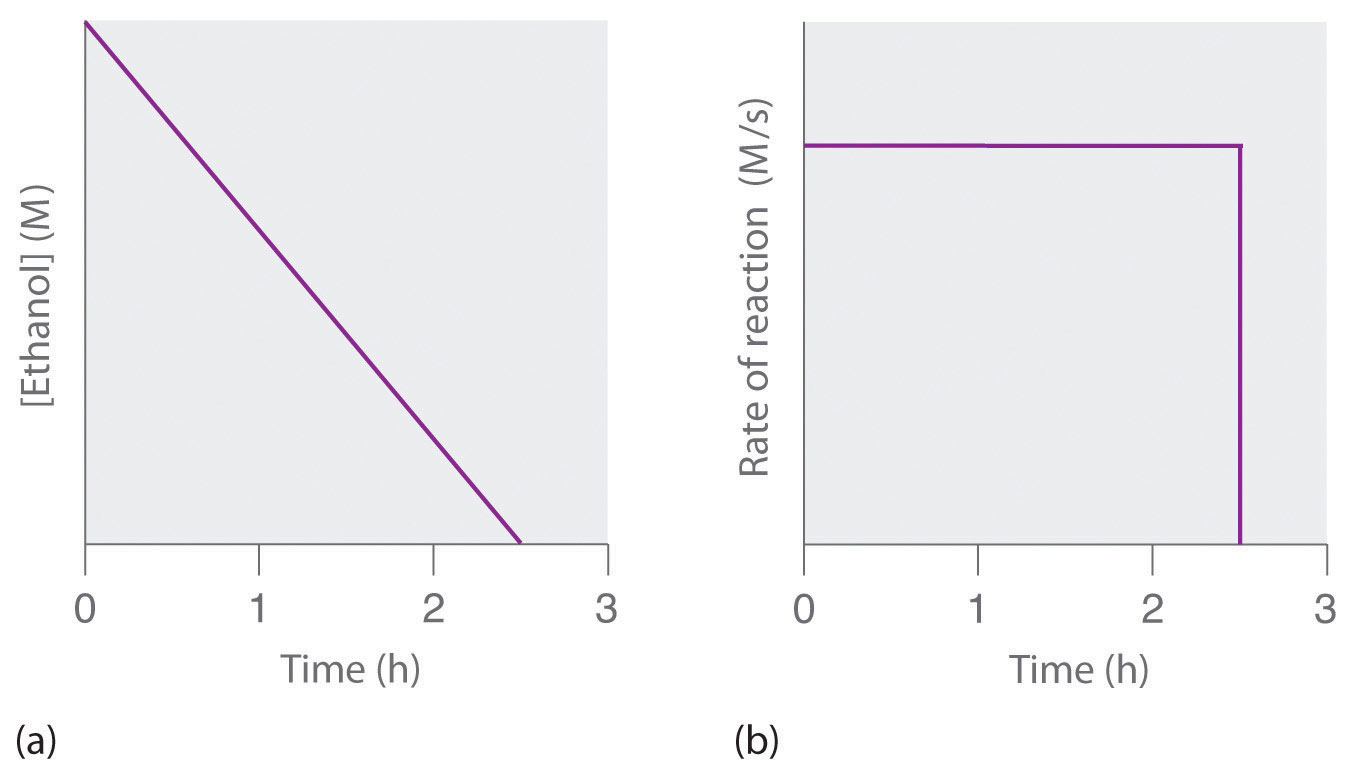
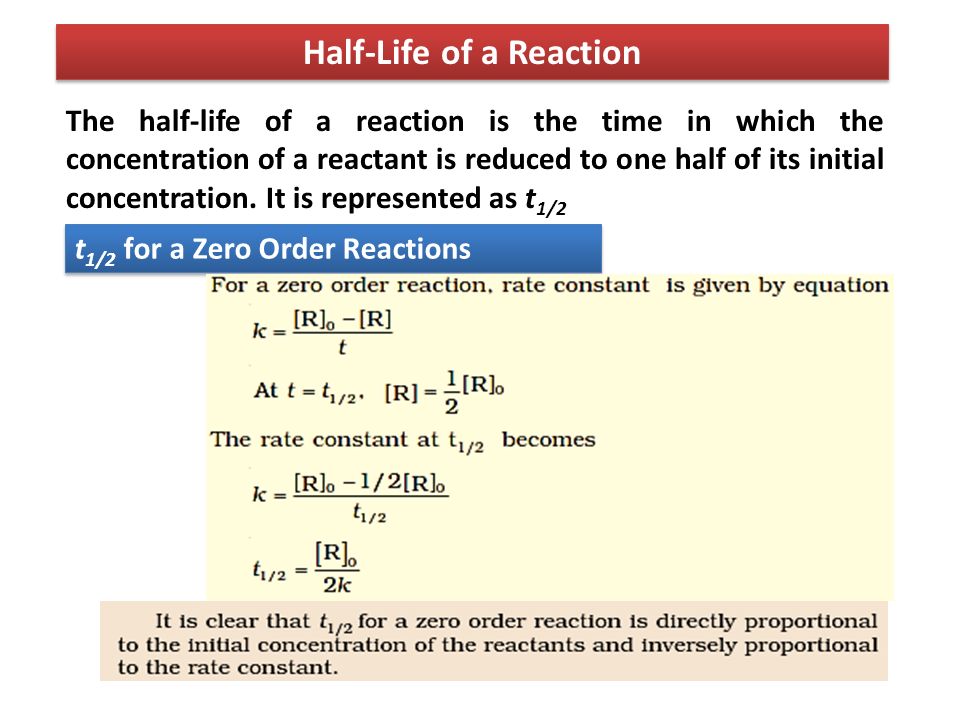
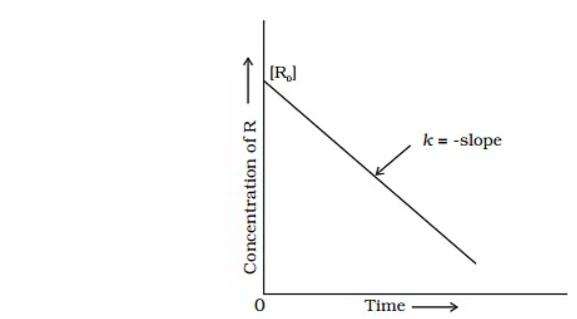
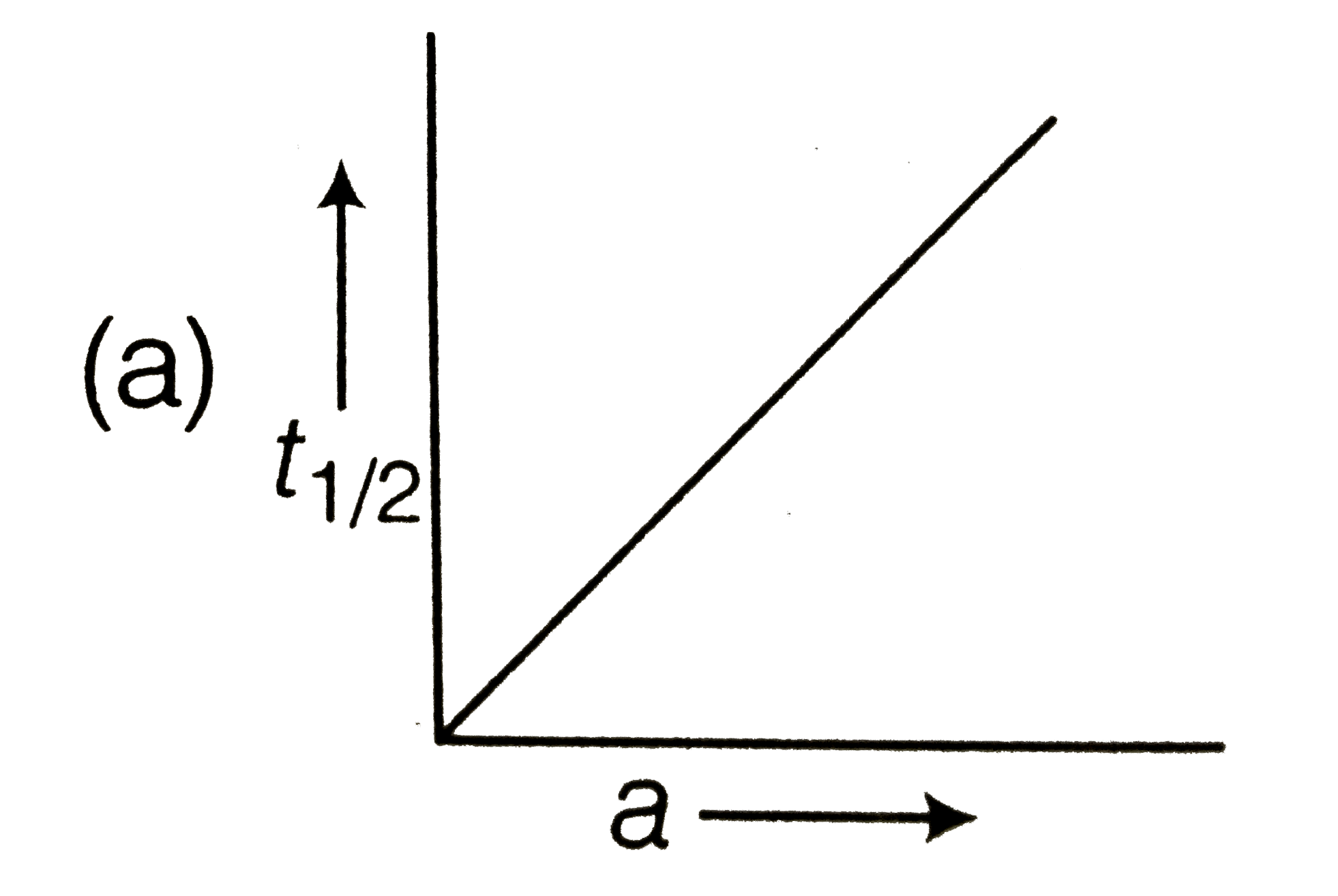
For a zero order reaction t 1/2 is proportional to initial concentration A 0t 1/2∝A 0t 1/2= 2kA 0The mathematical expression that can be employed to determine the halflife for a zeroorder reaction is, t1/2 = R 0/2k For the firstorder reaction, the halflife is defined as t1/2 = 0693/kExpress your answer with the appropriate units Halflife equation for firstorder reactions t1/2=0643k where t1/2 is the halflife in seconds (s), and k is the rate constant in inverse
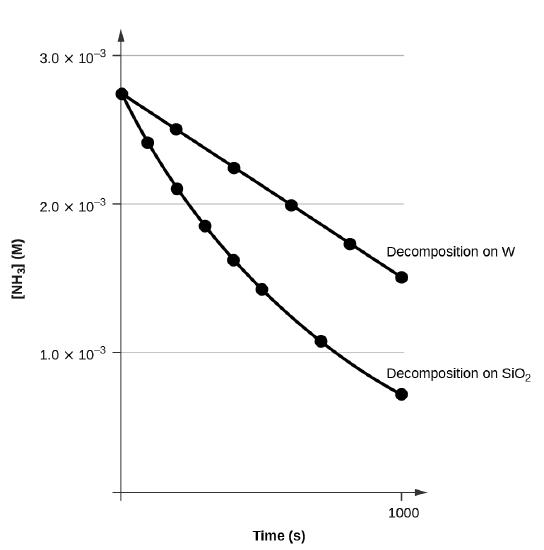
Kinetic theory states that minute particles of all matter are in constant motion and that the temperature of a substance is dependent on the velocity of this motion Increased motion isK = 1/2t 1/(ax) 21/a2 PSEUDOZERO ORDER REACTION In solid state, may drug decomposes by pseudo zero order ie reaction between drug and moisture in solid dosage form The systemHalflife equation for firstorder reactions t1/2 = 0693/k where t1/2 is the halflife in seconds (s), and k is the rate constant in inverse seconds (s1) What is the halflife of a first
T1/2 for zero order reaction formulaのギャラリー
各画像をクリックすると、ダウンロードまたは拡大表示できます
 |  |  |
 |  |  |
 |  | 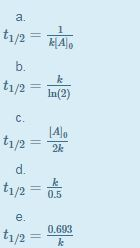 |
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 | ||
 |  |  |
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 | 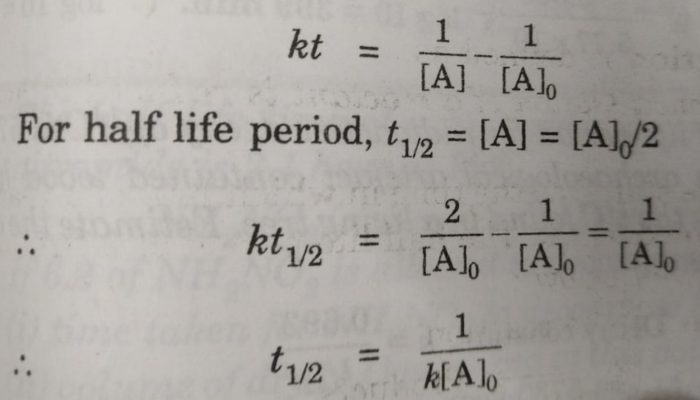 |  |
 |  | |
 |  |  |
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
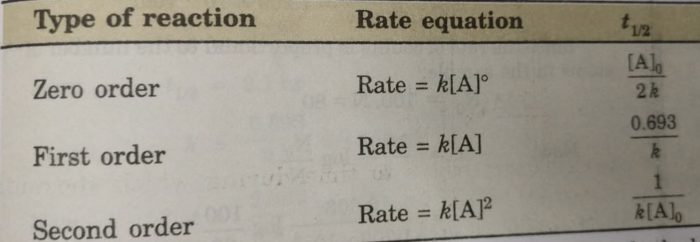 |  |  |
 |  | |
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 | ||
 |  | |
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  | |
 | ||
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 | 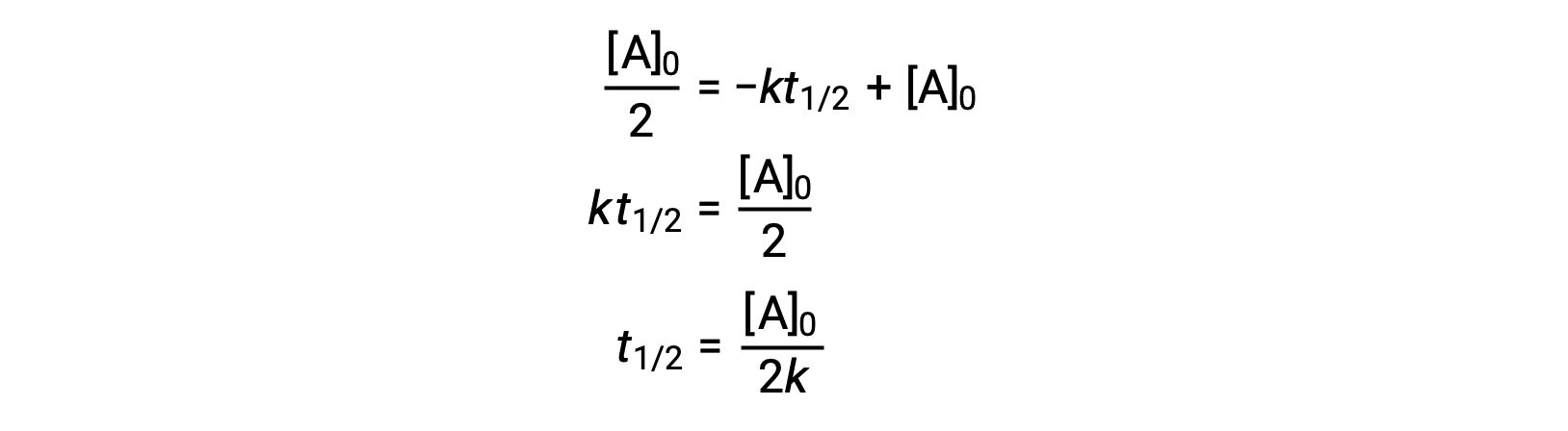 | |
 |  |  |
 |  |  |
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 | ||
 |  |  |
 |  |  |
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  | |
 |  |  |
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  | |
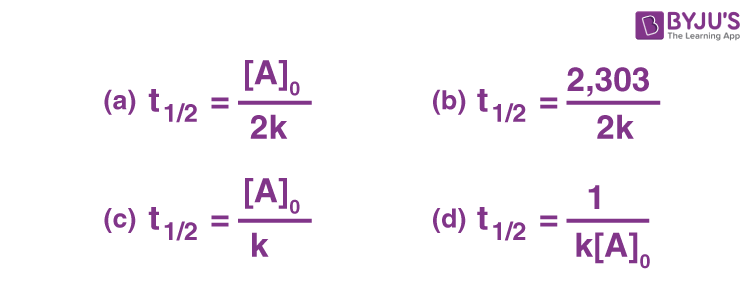 |  |  |
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
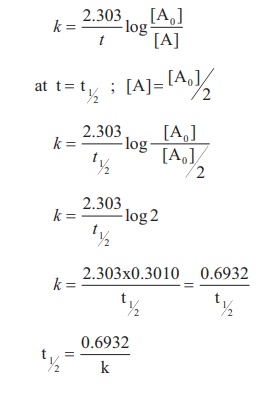 |  | |
 |  |  |
「T1/2 for zero order reaction formula」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |
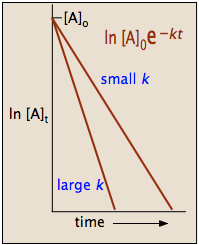
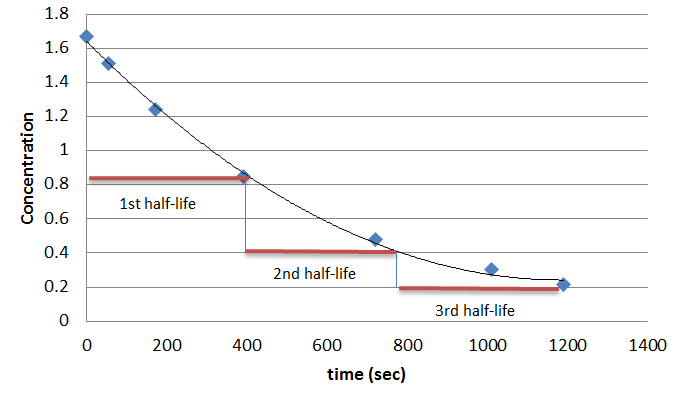
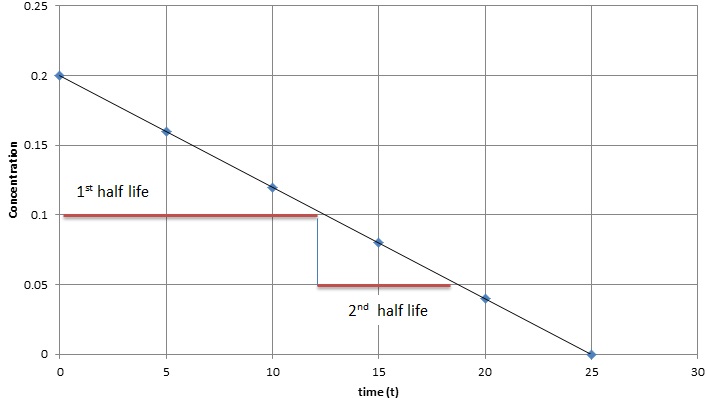
\text {reaction}\;\text {order}=\text {x}\text {y}=1\frac {1} {2}=\frac {3} {2} reaction order = xy = 1 21 = 23 The reaction is firstorder in hydrogen, onehalforder in bromine, and \frac {3} {2} 23The half life is given the symbol t 1/2 to denote that it is the time at which the concentration of reactant is one half its initial value For the first order reaction, you can plug the definition of the
Incoming Term: t1/2 for zero order reaction formula,




コメント
コメントを投稿